Cell Biology
Cut the CAT tails off and save mitochondria
26.06.2019
Protein aggregates are toxic for mitochondrial function, and thus disrupt the supply of chemical energy to their host cells. The labs of Roland Beckmann and Walter Neupert (Biomedical Center) have characterized a protein complex that prevents the build-up of such deposits in the organelles.
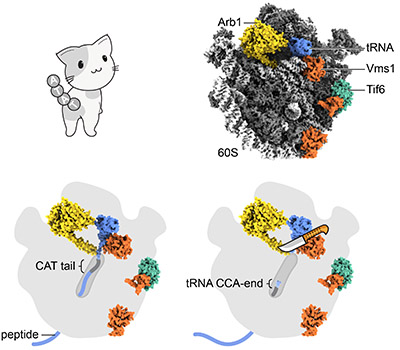
Top row, a cat with C-terminal alanyl and threonyl residues (CAT tail) looking at the molecular model of Vms1(Q295L)-60S complex including Arb1 (a pre-cleavage state of peptidyl-tRNA). Bottom left, cartoon of the 60S transverse section exposing the nascent peptide in the ribosomal tunnel. Bottom right, mechanistic model of the nucleolytic release of peptide by the wild-type Vms1 with the aid of Arb1. Image: T. Su
Mitochondria are the power stations that supply the cells of higher organisms with the chemical energy necessary for their metabolism and maintenance. In addition, the biosynthesis of many essential metabolites takes place in these organelles. Therefore, any perturbations in their function – such as abnormal accumulations of misfolded proteins – must be rapidly detected and repaired. Research teams led by LMU’s Walter Neupert (Biomedical Center) and Roland Beckmann (Gene Center) in cooperation with Toshifumi Inada (Sendai University, Japan) have now elucidated one of the mechanisms by which the cell inhibits the formation of toxic aggregates of proteins destined for the mitochondria, which could otherwise cut off the supply of energy for indispensable cellular functions.
The new findings appear in the journal Nature.
More information please visit LMU.de/news
Original Publication:
Structure and function of Vms1 and Arb1 in RQC and mitochondrial proteome homeostasis.
Su T, Izawa T, Thoms M, Yamashita Y, Cheng J, Berninghausen O, Hartl FU, Inada T, Neupert W, Beckmann R.
Nature. 2019 Jun 12. doi: 10.1038/s41586-019-1307-z.

