COVID-19
Researchers at LMU and PEI develop safe and effective RNA replicon vaccine
04.05.2021
Sustainable control of the current COVID-19 pandemic requires effective and safe vaccines. While the front-runner mRNA vaccines from BioNTech/Pfizer and Moderna seem to be safe, concerns exist with a number of virus-vectored vaccines in terms of safety or efficacy. Scientists around Alexandru Hennrich and Karl-Klaus Conzelmann (Max von Pettenkofer Institute and Gene Center of Ludwig-Maximilians-Universität) (LMU), and Christian Pfaller (Research unit pathogenesis of airway viruses at the Paul-Ehrlich-Institut (PEI), have joint to develop an RNA replicon vaccine which is highly efficient in animal models.
 The novel vaccine consists of a self-replicating RNA (replicon) derived from an animal rhabdovirus, vesicular stomatitis virus (VSV). The replicon encodes the part of the Coronavirus spike protein, which is the most important for eliciting a protective immunity. The replicons are enwrapped artificially into a VSV envelope for delivery of the RNA into cells where it can produce the protective antigen.
The novel vaccine consists of a self-replicating RNA (replicon) derived from an animal rhabdovirus, vesicular stomatitis virus (VSV). The replicon encodes the part of the Coronavirus spike protein, which is the most important for eliciting a protective immunity. The replicons are enwrapped artificially into a VSV envelope for delivery of the RNA into cells where it can produce the protective antigen.
A peculiarity of the vaccine is the design and presentation of the antigen. While in current vaccines whole coronavirus spike protein is being used as an antigen, the VSV replicon encodes a chimeric transmembrane “minispike”, presenting only the receptor binding domain (RBD) of the spike protein. As most of the antibodies protecting against COVID-19 are targeted to the RBD, the design of the minispike avoids induction of non-necessary and potentially harmful immune reponses. The chimeric antigen is presented by the replicon vaccine on the cell surface and stimulates production of antibodies which can block infection by the natural SARS-CoV-2 coronavirus.
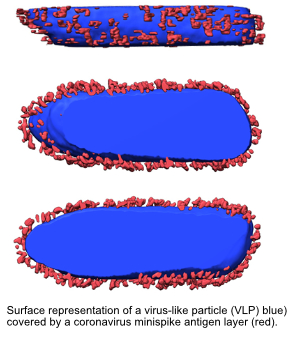
An additional key feature of the minispike is that its membrane part is similar to a rhabdovirus glycoprotein, such that the RNA replicon in infected cells can be mobilized again, such that noninfectious virus-like particles (VLPs) decorated with minispike are released, which can further stimulate the immune system to produce antibodies. The vaccine is thus a combination of classical vector- and VLP-vaccines. As revealed in animal experiments, this 2-in-1 vaccination leads to production of high antibody levels, which are sufficient to completely protect transgenic animals from COVID-19 disease. A second (boost) vaccination as recommended for most conventional vaccines is thus not required.
The authors, consider the new VSV-minispike vaccine approach suitable for immunization of immune suppressed patient, and as a promising platform for future development of vaccines against pathogens for which no appropriate vaccines are available so far. The findings were published in PLoS Pathogens.
Original Publication:
Safe and effective two-in-one replicon-and-VLP minispike vaccine for COVID-19: Protection of mice after a single immunization
Hennrich AA, Sawatsky B, Santos-Mandujano R, Banda DH, Oberhuber M, Schopf A, Pfaffinger V, Wittwer K, Riedel C, Pfaller CK, Conzelmann KK.
PLoS Pathog 17(4): e1009064. https://doi.org/10.1371/journal.ppat.1009064

