DNA repair
A ubiquitin switch controls autocatalytic inactivation of the DNA–protein crosslink repair protease SPRTN
22.12.2020
Julian Stingele and his team discover the components and principles of a ubiquitin switch, which negatively regulates SPRTN. They could show that monoubiquitylation induces inactivation of the enzyme by triggering autocatalytic cleavage in trans while also priming SPRTN for proteasomal degradation in cis.
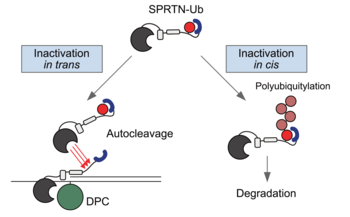 Repair of covalent DNA–protein crosslinks (DPCs) by the metalloprotease SPRTN prevents genome instability, premature aging and carcinogenesis. SPRTN is specifically activated by DNA structures containing single- and double-stranded features, but degrades the protein components of DPCs promiscuously and independent of amino acid sequence. This lack of specificity is useful to target diverse protein adducts, however, it requires tight control in return, in order to prohibit uncontrolled proteolysis of chromatin proteins. Julian Stingele and his team discover the components and principles of a ubiquitin switch, which negatively regulates SPRTN. They demonstrate that monoubiquitylation is induced in an E3 ligase-independent manner and, in contrast to previous assumptions, does not control chromatin access of the enzyme. Data obtained in cells and in vitro reveal that monoubiquitylation induces inactivation of the enzyme by triggering autocatalytic cleavage in trans while also priming SPRTN for proteasomal degradation in cis. Finally, they show that the deubiquitylating enzyme USP7 antagonizes this negative control of SPRTN in the presence of DPCs. The new findings are published in the journal Nucleic Acids Research.
Repair of covalent DNA–protein crosslinks (DPCs) by the metalloprotease SPRTN prevents genome instability, premature aging and carcinogenesis. SPRTN is specifically activated by DNA structures containing single- and double-stranded features, but degrades the protein components of DPCs promiscuously and independent of amino acid sequence. This lack of specificity is useful to target diverse protein adducts, however, it requires tight control in return, in order to prohibit uncontrolled proteolysis of chromatin proteins. Julian Stingele and his team discover the components and principles of a ubiquitin switch, which negatively regulates SPRTN. They demonstrate that monoubiquitylation is induced in an E3 ligase-independent manner and, in contrast to previous assumptions, does not control chromatin access of the enzyme. Data obtained in cells and in vitro reveal that monoubiquitylation induces inactivation of the enzyme by triggering autocatalytic cleavage in trans while also priming SPRTN for proteasomal degradation in cis. Finally, they show that the deubiquitylating enzyme USP7 antagonizes this negative control of SPRTN in the presence of DPCs. The new findings are published in the journal Nucleic Acids Research.
Original Publication:
A ubiquitin switch controls autocatalytic inactivation of the DNA–protein crosslink repair protease SPRTN
Zhao S, Kieser A, Li H-Y, Reinking HR, Weickert P, Euteneuer S, Yaneva D, Acampora AC, Götz MJ, Feederle R and Stingele J.
Nucleid Acids Research. 2020 Dec 26; doi: 10.1093/nar/gkaa1224

