Structural Biology
Special delivery
31.01.2020
Bulky globular proteins require specialized transport systems for insertion into membranes. Roland Beckmann and his team have determined the structure of such a system for the first time, and propose that it exploits the principle of the airlock.
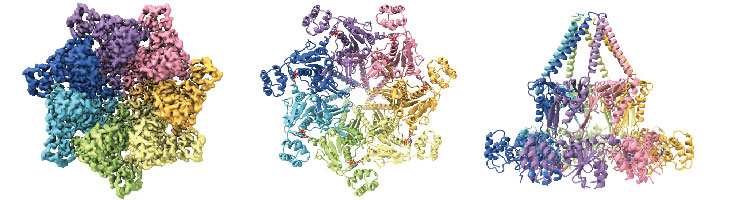
Top (left and middle) and side (right) views of Bcs1: The Bcs1 AAA-ATPase, an integral membrane protein of the inner mitochondrial membrane forms an unusual heptameric ring. Source: Kater et al., NSMB 2020
Many proteins that are essential for cell viability must be transported across the membranes of intracellular organelles – such as mitochondria – in order to reach their sites of action. As a rule, transmembrane transport takes place before the newly synthesized protein has folded into its final functional shape, as the unfolded form can be extruded through a relatively narrow pore. The passage of folded proteins is a more difficult operation. They take up more space, so the pore must be correspondingly larger. Bacteria and chloroplasts utilize dedicated tunnels for this purpose. Although they are evolutionarily related to bacteria, mitochondria lack this type of aperture. Nevertheless, they manage to transfer one important protein in its folded state across their inner membrane. Now, researchers led by Professor Roland Beckmann at the LMU Gene Center have reported evidence which suggests that mitochondria accomplish this challenging feat by making use of the airlock principle. The structural biologist Beckmann and his colleagues describe their findings in a paper that appears in the journal Nature Structural and Molecular Biology.
More information please visit LMU.de/news
Original Publication:
Structure of the Bcs1 AAA-ATPase suggests an airlock-like translocation mechanism for folded proteins.
Kater L, Wagener N, Berninghausen O, Becker T, Neupert W, Beckmann R.
Nature Structural and Molecular Biology 27, 142–149 (2020). https://doi.org/10.1038/s41594-019-0364-1

