DNA structure-specific cleavage of DNA-protein crosslinks by the SPRTN protease
NMR analysis reveals that specificity is achieved by a bipartite strategy
26.08.2020
Julian Stingele and his team in cooperation with the labs of Lucas Jae and Michael Sattler show that the protease SPRTN degrades DNA-protein crosslinks in a DNA structure-specific manner, which restricts cleavage to biologically relevant scenarios. NMR analysis reveals that specificity is achieved by a bipartite strategy relying on two DNA binding interfaces that recognize single- and double-stranded features within the substrate.
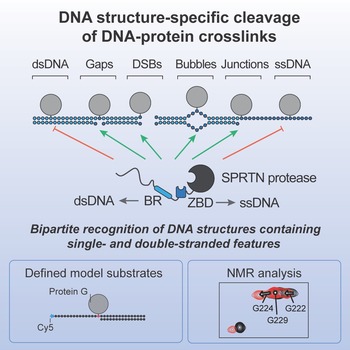 The repair of covalent DNA-protein crosslinks (DPCs) by DNA-dependent proteases has been shown to be an essential genome maintenance mechanism. This process is necessary for cellular viability and tumor suppression. However, how the cleavage activity of the protease is restricted to the crosslinked protein while leaving surrounding chromatin proteins unharmed has remained unknown. In the study which appears in the journal Molecular Cell, the authors use defined DPC model substrates to show that the DPC protease SPRTN displays strict DNA structure-specific activity. The DNA context is coupled to the activation of the enzyme, which limits the effect of SPRTN closely to biologically relevant scenarios.
The repair of covalent DNA-protein crosslinks (DPCs) by DNA-dependent proteases has been shown to be an essential genome maintenance mechanism. This process is necessary for cellular viability and tumor suppression. However, how the cleavage activity of the protease is restricted to the crosslinked protein while leaving surrounding chromatin proteins unharmed has remained unknown. In the study which appears in the journal Molecular Cell, the authors use defined DPC model substrates to show that the DPC protease SPRTN displays strict DNA structure-specific activity. The DNA context is coupled to the activation of the enzyme, which limits the effect of SPRTN closely to biologically relevant scenarios.
Graphical abstract. Source: Molecular Cell 2020.

