Keppler Lab - Research
SARS-CoV-2: Cellular regulators, antiviral drugs and neutralization
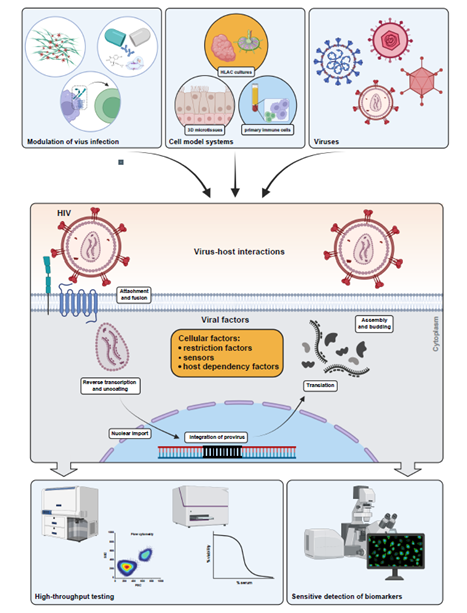 In the fight against COVID-19 we are testing novel antiviral approaches, including peptide-linked siRNAs, to reduce viral replication and disease burden in ex vivo models and in vivo.
In the fight against COVID-19 we are testing novel antiviral approaches, including peptide-linked siRNAs, to reduce viral replication and disease burden in ex vivo models and in vivo.
Based on newly developed cell models, we are aiming to identify cellular pathways involved in SARS-CoV-2 replication and virus-induced cell death. To this end, we are employing an unbiased genetic survival screen to identify important cellular factors. In addition, we are performing a high-throughput screen using a complex small molecule library to identify lead compounds for drug development.
In addition, we are elucidating the neutralizing activity of sera from COVID-19 patients and vaccinated individuals to SARS-CoV-2 variants of concern using a newly developed high-throughput assay based on authentic virus.

