Ferraz Lab - Research
- Research
- Extracellular vesicles in reproduction
- Bio-printing and microfluidics
- Microplastics and fertility
Extracellular vesicles in reproduction
Since its introduction over 35 years ago, in vitro fertilization (IVF) has assumed great promise for infertile couples, but success rates have remained only ~30%. It is well-established that embryos produced in vitro possess reduced developmental competence and can display epigenetic (non-genetic influences in gene expression) changes. Despite the known links between in vitro embryo manipulation and perturbed epigenetic, very little is known about how to prevent them. The maternal-embryonic interface has been defined as the site of contact between the embryo and the maternal oviduct (Fallopian tube) and endometrium. A newer view is that this interface is even more expansive than previously realized, on account that great quantities of extravesicular material shed from the maternal reproductive tract. Intercellular communication via extracellular vesicles (EVs) represents an evolutionarily conserved mechanism of communication, yet one we know little about.
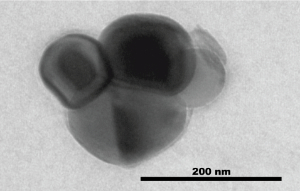
Transmission Electron Microscopy image of extracellular vesicles collected from oviductal fluid.
EVs are membrane encapsulated particles containing regulatory molecules that contribute to cell-cell communication by carrying proteins, peptides, RNA species (microRNAs, mRNAs, and long non-coding RNAs), lipids, and DNA fragments. EVs have been isolated from prostate, epididymal, uterine, follicular and oviductal fluids. Yet, only a hand full of studies have investigated the role of oviductal EVs on gamete maturation and embryo production. Moreover, to date, there are no studies on the impact of oviductal and endometrial EVs, on the embryo (epi)genome. In vitro studies suggest increased development and pregnancy success after embryo transfer when oviductal EVs were used during IVF. However, major gaps remain in our understanding of EVs, in part because there is a paucity of models available to study reproductive EVs in vitro. In our lab, we close some of these gaps by using a new in vitro model of an oviduct-endometrium-on-a-chip, which can be used as an in vitro source of EVs and be genetically manipulated to investigate which EVs factors, can influence gamete function and interaction and embryo epigenetic. To move toward the long term goal of improving the outcome for embryos manipulated in vitro, our overall aim is to study how the maternal-embryonic communication through the use of in vitro and in vivo produced EVs, can improve gamete interaction and embryo quality.
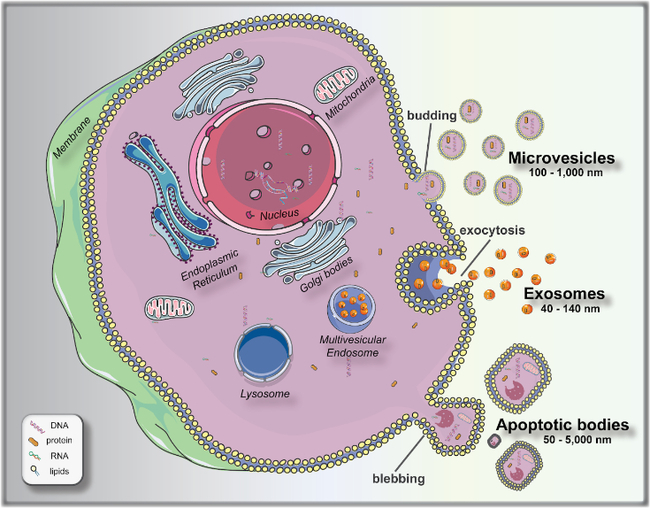
Biogenesis and release of extracellular vesicles (EVs). Microvesicles bud directly from the plasma membrane; exosomes are formed by budding of small vesicles into early endosomes and multivesicular endosomes, which are released by exocytosis; and apoptotic bodies are released by outward blebbing of apoptotic cells. From Talebjedi et al., 2021.
Publications:
Oviductal Extracellular Vesicles Improve Post-Thaw Sperm Function in Red Wolves and Cheetahs.
Ferraz MAMM, Nagashima JB, Noonan MJ, Crosier AE, Songsasen N.
Int J Mol Sci. 2020 May 25;21(10):3733. doi: 10.3390/ijms21103733. PubMed
Cat follicular extracellular vesicles contain proteins regulating cell signaling pathways that enhance meiotic resumption of cat vitrified oocytes.
Ferraz MAMM, Fujihara M, Nagashima JB, Noonan MJ, Inoue-Murayama M, Songsasen N.
Sci Rep. 2020 May 25;10(1):8619. doi: 10.1038/s41598-020-65497-w. PubMed
Oviductal extracellular vesicles interact with the spermatozoon's head and mid-piece and improves its motility and fertilizing ability in the domestic cat.
Ferraz MAMM, Carothers A, Dahal R, Noonan MJ, Songsasen N.
Sci Rep. 2019 Jul 1;9(1):9484. doi: 10.1038/s41598-019-45857-x. PubMed
Reviews:
Exploiting Microfluidics for Extracellular Vesicle Isolation and Characterization: Potential Use for Standardized Embryo Quality Assessment.
Talebjedi B, Tasnim N, Hoorfar M, Mastromonaco GF, Ferraz MAMM.
Front Vet Sci. 2021 Jan 5;7:620809. doi: 10.3389/fvets.2020.620809. PubMed

