Wolf Lab - Research
Role of the ERBB system in tissue homeostasis and tumorigenesis
ERBB receptors are receptor tyrosine kinases (RTK) and important regulators of tissue development, homeostasis and tumorigenesis. The ERBB receptor family consists four cell surface receptors: the epidermal growth factor receptor (EGFR, ERBB1, HER1), ERBB2 (neu, HER2), ERBB3 (HER3), and ERBB4 (HER4).
EGFR is expressed in a wide variety of organs and it can act as an oncoprotein in many types of cancer; and therefore is EGFR an important target in human cancer therapy. ERBB2 is an orphan receptor without any known ligand and ERBB3 has a dead kinase domain. Notably, ERBB2/ERBB3 heterodimers show the strongest mitogenic activity of all ERBB receptor combinations, and their frequent presence in tumors makes them potential targets for a cancer therapy. ERBB4 the last member of the family, is not one of the major players in tumor biology, but in some tumors ERBB4 can act as a backup system for other ERBB receptors.
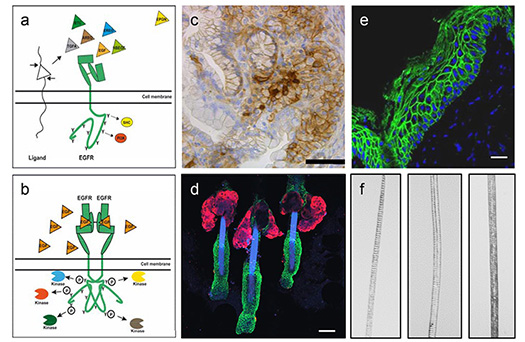
a) Drawing of an inactivated EGFR and its ligands. B) Drawing of an activated EGFR. c) Immunohistochemistry for betacellulin in murine PanINs. d) Whole mounts of murine hair follicles (blue=DAPI, red=SCD, green=K14). e) Immunofluorescence of EGFR in human epidermis (blue=DAPI, green=EGFR). f) Different murine hair types.
One focus is to understand the role of the ERBB system, in particular the both oncoproteins ERBB2 and ERBB3 in non-melanoma skin cancer (NMSC), but also during skin homeostasis. NMSC like squamous cell carcinoma (SCC) and the basal cell carcinoma (BCC) are the most common cancer amongst Caucasians; more than 3 million cases are recorded per year just in the United States. SCC develops from keratinocytes and is mainly caused by UV light exposure and it usually occurs on sun-exposed areas of the body, such as face and neck. Different environmental conditions, as UV light, or diseases can disturb the ERBB signaling cascade this result in positive and negative feedback loops of the system. Recently, the leucine-rich repeats and immunoglobulin-like domains (LRIG) family attracted our attention because of their influence on these feedback mechanisms. At the moment we are focused on LRIG1-3 proteins and their interactions with the ERBB receptors in the skin during UV light radiation and tumorigenesis.
The second main aim is to understand the ERBB signaling in the gastrointestinal tract, at the moment with an especial focus on the exocrine pancreas and the pancreatic ductal adeno carcinoma (PDAC). PDAC is the predominant form of pancreatic cancer with a poor prognosis. The lack of early clinical symptoms leads to late diagnoses at stages when the tumor has already metastasized, which makes PDAC one of the lethal cancers in the United States and Europe. Since pancreatic cancer shows poor response to many therapies, the 5-year-survival rate only remains 4-6%. The pathological mechanism of PDAC involves a dysfunction of the ERBB family. We are studying the role of betacellulin (BTC), one of the eleven ligands of the ERBB receptor family, who can directly activate EGFR and ERBB4. Therefore, we are using mice carrying a conditional KrasG12D allele under the control of the pancreas specific Ptf1 promoter. Because over 95% of all human patients with PDAC carry a point mutation in the KRAS gene inducing a constitutively active form of the KRAS protein (KRASG12D) the KrasG12D mouse is a great model to study PDAC. We discovered that an additional overexpression of BTC in these mice leads to an earlier onset and drastically faster progression of PDAC and a very early mortality rate.
First of all our research should help to understand the ERBB signaling in healthy and tumorigenic tissue, and afterwards we want to use this knowledge to target the ERBB system during cancer therapy.

