Hopfner Lab - Research
- Research
- Chromatin Remodeller
- Innate Immunity
- Therapeutic Protein Engineering
- OPSYON Spin-off Project
Therapeutic Protein Engineering
Monoclonal antibodies have become a powerful tool in cancer immunotherapy and many antibody-based approaches and novel classes of engineered antibody constructs have been proven successful to eradicate cancer cells. Although our immune system has great potential to eliminate tumors, cancer progression is often accompanied by profound immune suppression that interferes with an effective anti-tumor response and tumor elimination. Thus, the recent development of monoclonal antibodies targeting immune regulatory checkpoints, which are often utilized by cancer cells to escape immune recognition and gain immune resistance, is a highly promising strategy for treating different types of cancers. The development of monoclonal antibodies targeting immune regulatory checkpoint molecules such as PD-1 (programmed cell death-1), its ligand PD-L1 (programmed death-ligand 1) or the “don’t eat me” receptor CD47 has reinvigorated the field of immunotherapy and convincingly demonstrated their immense potential in cancer therapy and clinical efficacy.
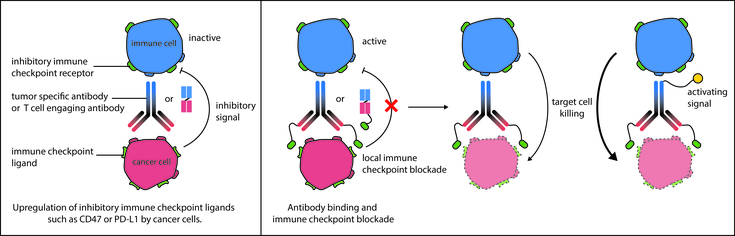
Figure: Schematic overview of mechanism of action. Monoclonal antibodies or derivatives thereof induce target cell killing by activating immune cells through a local immune checkpoint blockade and/or immune cell activating signals.
Our research is driven by the vision to use our expertise in protein science to improve antibody-based cancer immunotherapy and to optimize the molecules for the medical needs of cancer patients. The main goal of our projects is to develop therapeutic molecules that provide cancer patients a long-term possibility for progression free survival with a low risk of side effects during treatment. To this end, we engineer therapeutic antibodies and derivatives thereof to target a specific tumor antigen with a simultaneous activation or inhibition of immune checkpoints. By this approach, we locally restrict the immune response to the tumor cells and consequently reduce the likelihood of systemic side effects or immune-related adverse events (irAEs).
Additionally, our novel multi-specific antibody formats further allow for the engagement and activation of effector cells of the innate and/or adaptive immune system. Finally, we analyze our molecules on patient samples and in in vivo models in cooperation with the group of Marion Subklewe, the spin-off project OPSYON and other clinical collaborators validate the potential to treat hematological and solid malignancies.
Publications:
SIRPα-antibody fusion proteins stimulate phagocytosis and promote elimination of acute myeloid leukemia cells.
Ponce LP, Fenn NC, Moritz N, Krupka C, Kozik JH, Lauber K, Subklewe M, Hopfner KP.
Oncotarget. 2017 Feb 14;8(7):11284-11301. PubMed
CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells.
Schiller CB, Braciak TA, Fenn NC, Seidel UJ, Roskopf CC, Wildenhain S, Honegger A, Schubert IA, Schele A, Lämmermann K, Fey GH, Jacob U, Lang P, Hopfner KP, Oduncu FS.
Oncotarget. 2016 Dec 13;7(50): 83392-83408. PubMed
Dual-targeting triplebody 33-3-19 mediates selective lysis of biphenotypic CD19+ CD33+ leukemia cells.
Roskopf CC, Braciak TA, Fenn NC, Kobold S, Fey GH, Hopfner KP, Oduncu FS.
Oncotarget. 2016 Apr 19;7(16):22579-89. PubMed
Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies.
Niederfellner G, Lammens A, Mundigl O, Georges GJ, Schaefer W, Schwaiger M, Franke A, Wiechmann K, Jenewein S, Slootstra JW, Timmerman P, Brännström A, Lindstrom F, Mössner E, Umana P, Hopfner KP, Klein C.
Blood. 2011 Jul 14;118(2):358-67. PubMed

